Marcus Tius and colleague have reported in ACIE on the asymmetric synthesis of rocaglamide.

Archive for March, 2015
Rocaglamide synthesis
Posted by naturalproductman on March 31, 2015
Posted in Asymmetric, Named Reactions, Nazarov cyclization, Total Synthesis | Leave a Comment »
Chlorinated vinyl epoxide ring opening with TMS chloride
Posted by naturalproductman on March 29, 2015
Erick Carreira and co-workers have reported in Organic Letters on a chlorinated vinyl epoxide ring opening using TMS chloride.

OL paper
Posted in Cascade Reactions, Methodology | Leave a Comment »
Perforalactones A and B
Posted by naturalproductman on March 29, 2015
Xiao Jiang Hao and co-workers from the Chinese Academy of Sciences have reported in ACIE on the isolation of a quassinoid, perforalactone B, which had antagonistic activity with an IC50 of 1.26 nM against nicotinic acetylcholine receptor, which means it’s a good pesticide.

Posted in Uncategorized | Leave a Comment »
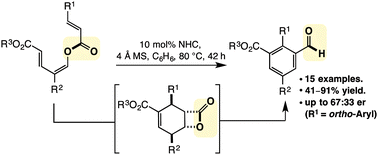
Substituted benzaldehydes via NHCs
Posted by naturalproductman on March 25, 2015
David Lupton and co-workers at Monash University have reported in Chemical Science on a method to synthesize substituted benzenes from acyclic precursors.
Chemical Science paper
Posted in Carbene, Cascade Reactions, Methodology | Leave a Comment »
Cascade reaction towards furans
Posted by naturalproductman on March 25, 2015
Stephen Clark and co-workers from the University of Glasgow have reported in ACIE on a neat cascade reaction.

Posted in Uncategorized | Leave a Comment »
Corvol ethers A and B biosynthesis
Posted by naturalproductman on March 25, 2015
Jeroen Dickschat and co-workers at Technische Universität Braunschweig have reported in ACIE on some terpene biosynthesis by characterizing a sesquiterpene cyclase enzyme.
Posted in Biosynthesis, Enzymes, Sesquiterpenes | Leave a Comment »
Deethylibophyllidine and limaspermidine syntheses
Posted by naturalproductman on March 25, 2015
Chun-An Fan and co-workers from Lanzhou University have reported in JACS on the synteses of deethylibophyllidine and limaspermidine.

Posted in Alkaloids, Total Synthesis | Leave a Comment »
Something to scratch your head about…
Posted by naturalproductman on March 25, 2015
Here’s a weird one from Joseph Ready’s lab at UT Southwestern. Nigricanoside was isolated and reported in JACS in 2007 and was reported to be very biologically active against human breast cancer MCF-7 cells with an IC50 of 3 nM. The research lab had synthesized the molecule and found no biological activity at all, but I looked for the data on biological activity in the supporting information and did not see anything with the biological testing data. Since the strong claim of the lack of activity would make the original isolation paper not as interesting (afterall, most people are mainly interested in the biological activity of natural products besides the exotic structures in the perspective of a synthetic chemist), it would probably have been good for the group to make that data available in their publication. But a counter argument is what is so exciting about negative data? The original isolation paper in JACS has an image on mitotic arrest of breast cancer cells (Figure 2). The biological testing is mentioned in the acknowledgments of the Chemical Science paper with the names of people from two schools.
Another example of this kind of difference in biological activity between a synthesized natural product vs. the original isolation report is with berkelic acid isolated by Andrea Stierle and co-workers from an abandoned copper mine. Barry Snider’s lab had synthesized this polyketide and found no biological activity – the biological data from the NCI screen panel was reported in the supporting information (pages 23-25 in SI) of the JOC paper (Snider group).
Posted in Cancer, Total Synthesis | Leave a Comment »
[2+2] via oxazaborolidine
Posted by naturalproductman on March 22, 2015
M. Kevin Brown and co-workers at Indiana University have reported in JACS on an asymmetric [2+2] using an oxazaborolidine catalyst.

Posted in Asymmetric, Cycloaddition, Methodology, [2+2] | Leave a Comment »
Dicarabrones A and B
Posted by naturalproductman on March 21, 2015
Yang Ye and co-workers from the Chinese Academy of Sciences have reported in Organic Letters on isolation of dicarabrones A and B.

Posted in Cancer, Lactones, Sesquiterpenes | Leave a Comment »