Archive for the ‘Diels-Alder’ Category
Posted by naturalproductman on May 7, 2016
Paul Race and co-workers from the University of Bristol have reported in JACS on the details of how an enzyme catalyzes a Diels-Alder reaction using crystal structures and enzyme assays. The enzyme is called spirotetronate cyclase AbyU and is key in forming the macrocyclic structure of abyssomicin C.
JACS paper
Posted in Diels-Alder, Enzymes | Leave a Comment »
Posted by naturalproductman on February 12, 2016
Scott Snyder and co-workers at the University of Chicago have reported in ACIE on a formal synthesis of gracilamine.

ACIE paper
Posted in Alkaloids, Diels-Alder, Named Reactions, retro-Diels-Alder, Total Synthesis | Leave a Comment »
Posted by naturalproductman on January 29, 2016
Michael Sherburn and co-workers from the Australian National University reported in JOC on a Diels-Alder cascade sequence.

JOC paper
Here’s an interesting related ACIE paper by the same group.
Posted in Cascade Reactions, Diels-Alder, Methodology, Named Reactions | Leave a Comment »
Posted by naturalproductman on January 27, 2016
Yandong Zhang and co-workers at Xiamen University have reported in Organic Letters on a synthesis of fusarisetin A using a one pot intramolecular Diels-Alder/Mukaiyama aldol reaction cascade sequence.
OL paper
Posted in Cascade Reactions, Diels-Alder, Named Reactions, One pot, Total Synthesis | Leave a Comment »
Posted by naturalproductman on January 22, 2016
John Gao and co-workers from UT San Antonio have reported in ACIE on the use of modularly designed organocatalysts (MDO) to effect a hetero-Diels-Alder/oxy-Michael cascade reaction.
ACIE paper
Posted in Cascade Reactions, Diels-Alder, Kinetic resolution, Methodology, Named Reactions, Organocatalytic, Oxy-Michael | Leave a Comment »
Posted by naturalproductman on January 19, 2016
John Porco and co-workers at Boston University have reported in JACS on the syntheses of sanggenons C and O.
JACS paper
Posted in Diels-Alder, Named Reactions, Polyketides, Total Synthesis | Leave a Comment »
Posted by naturalproductman on October 16, 2015
Xuegong She and co-workers from Lanzhou University have reported in Chemical Communications on a domino Diels-Alder transformation to form a polycyclic scaffold.
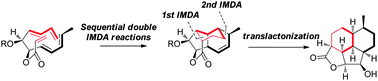
Chemical Communications paper
Posted in Cascade Reactions, Diels-Alder, Methodology, Named Reactions | Leave a Comment »
Posted by naturalproductman on October 16, 2015
Bo Liu and co-workers at Sichuan University have reported in JACS on the synthesis of atisane-type diterpenoids.

JACS paper
Posted in Diels-Alder, Diterpenoids, Methodology, Named Reactions, Total Synthesis | Leave a Comment »
Posted by naturalproductman on October 13, 2015
Thomas Hoye and colleague at the University of Minnesota have reported in Nature Chemistry on a Diels-Alder reaction to access paracaseolide.
Nature Chemistry paper
Posted in Biomimetic, Cascade Reactions, Diels-Alder, Methodology, Named Reactions, Total Synthesis | Leave a Comment »
Posted by naturalproductman on August 28, 2015
Rodrigo Andrade and co-workers from Temple University have reported in ACIE on the synthesis of

ACIE paper
Posted in Alkaloids, Diels-Alder, Dimer, Methodology, Named Reactions, Total Synthesis | Leave a Comment »




