Gregg Keaney and co-workers at H3 Biomedicine have reported in Org Lett on the synthesis of 6-deoxypladienolide D.

Archive for the ‘Suzuki-Miyaura’ Category
Synthesis of 6-Deoxypladienolide D, which binds to SF3B1
Posted by naturalproductman on October 28, 2014
Posted in Named Reactions, Polyketides, Suzuki-Miyaura, Total Synthesis | 1 Comment »
Enantiospecific Suzuki-Miyaura
Posted by naturalproductman on December 5, 2011
Michinori Suginome and co-workers at Kyoto University have published in JACS on the enantiospecificity of a Suzuki-Miyaura reaction and they show that inversion or retention can occur depending on the additive.

Posted in Asymmetric, cross coupling, Methodology, Palladium, Suzuki-Miyaura, Transition Metal, Zirconium | Leave a Comment »
Pyrrole access
Posted by naturalproductman on June 5, 2011
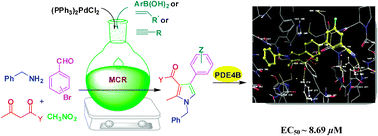
Manojit Pal and co-workers from the University of Hyderabad have reported in Chem Comm on a multi-component reaction to make substituted pyrroles.
Posted in Cascade Reactions, cross coupling, Methodology, Multi-Component Coupling, Palladium, Suzuki-Miyaura, Transition Metal | Leave a Comment »
Haouamine B Progress
Posted by naturalproductman on January 20, 2011
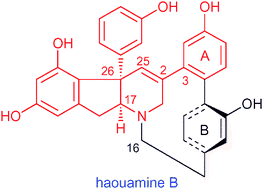
Yoshiki Morimoto and co-workers from Osaka City University have published in Chemical Communications on their progress towards haouamine B. Phil Baran and co-worker have published on a synthesis of haouamine A – however, haouamine B, which differs from the A form by a hydroxy group in one of the aryl rings (the one fused to the five membered ring), is less stable than A and has not been synthesized yet.
Posted in Alkaloids, cross coupling, Methodology, Palladium, Suzuki-Miyaura, Transition Metal | Leave a Comment »
Didemnaketal Synthesis
Posted by naturalproductman on October 29, 2010
Haruhiko Fuwa and co-workers from Tohoku University have published in Organic Letters on a synthesis of didemnaketal which has an IC50 value of 1 micromolar against HIV-1 protease. The spiroketal unit was accessed from a PPTS catalyzed condensation of an alcohol and a dihydropyran.

Posted in Cascade Reactions, cross coupling, Diseases, HIV, Methodology, Palladium, Polyketides, Spiroketals, Suzuki-Miyaura, Transition Metal | Leave a Comment »
Suzuki-Miyaura with Aryltriazenes
Posted by naturalproductman on July 13, 2010
Meiming Luo and co-workers from Sichuan University have published in the Beilstein Journal of Organic Chemistry on a Suzuki-Miyaura reaction that involves the use of a triazene compound, which couples with an arylboronic acid.

Posted in cross coupling, Methodology, Palladium, Suzuki-Miyaura, Transition Metal | Leave a Comment »
Indene Synthesis
Posted by naturalproductman on May 20, 2010
Mark Lautens and co-worker at the University of Toronto have published in Organic Letters on the use of a gem-dibromoalkene to form an indene ring and a new C-C bond via an intermolecular Suzuki/Heck reaction. Usually we see alkynes as precursors to these transition metal catalyzed indene formations, but this dibromo-olefin is another approach.

Posted in cross coupling, Methodology, Mizoroki-Heck, Palladium, Ring forming, Suzuki-Miyaura, Transition Metal | Leave a Comment »
Boron Enolate from Alkyne
Posted by naturalproductman on April 9, 2010
Tom Sheppard and co-workers from the University College in London have recently reported in JACS on an unconventional way to form boron enolates from alkynes – it basically looks like the addition of the -OH group accross the alkyne for a 6-endo-dig cyclization.

Posted in Chan Lam, Copper, cross coupling, Gold, Methodology, Named Reactions, Suzuki-Miyaura, Transition Metal | Leave a Comment »
Regioselective Suzuki-Miyaura
Posted by naturalproductman on March 31, 2010
Peter Langer and co-workers at the Universitat Rostock have recently published in Synlett on a regioselective Suzuki-Miyaura reaction that selectively couples a boronic acid to a specific triflate on an aryl ring. Their explanation had to do with the electronics.

Posted in cross coupling, Methodology, Palladium, Suzuki-Miyaura, Transition Metal | Leave a Comment »
Tandem Oxidation-Wittig
Posted by naturalproductman on February 17, 2010
Richard Taylor and co-workers at the University of York have recently published in Synlett on a tandem approach to synthesize dibromo-beta-substituted enone from an alpha hydroxy ketone. Hydrazine was condensed with the carbonyl and a Suzuki-Miyaura cross coupling followed to form a pyrazole ring.

Posted in Cascade Reactions, cross coupling, Methodology, Named Reactions, Palladium, Steroids, Suzuki-Miyaura, Transition Metal, Wittig | Leave a Comment »