Archive for the ‘Regioselective’ Category
Posted by naturalproductman on August 28, 2015
Takeo Kawabata and co-workers from Kyoto University have reported in ACIE on the synthesis of multifidosides through a regioselective acylation.

ACIE paper
Posted in Glycosides, Methodology, Regioselective, Total Synthesis | Leave a Comment »
Posted by naturalproductman on June 30, 2015
Matthias Beller and co-workers have reported in JACS on a ligand controlled regioselectivitiy switching of an alkoxycarbonylation of an allene substrate.
JACS paper
Posted in Methodology, Palladium, Regioselective, Transition Metal | Leave a Comment »
Posted by naturalproductman on April 12, 2015
Aaron Aponick and co-workers from the University of Florida have used a trimethylphosphine additive to improve the gold catalyzed spiroketalization for spirastrellolide.

OL paper
Posted in Diastereoselective, Gold, Methodology, Regioselective, Transition Metal | Leave a Comment »
Posted by naturalproductman on January 24, 2012
Kana Sureshan and co-workers from the Indian Institute of Science Education and Research have reported in Chemical Communications on the use of a sulfuric acid/silica gel catalyst to acylate the 2-hydroxy group of inositol.
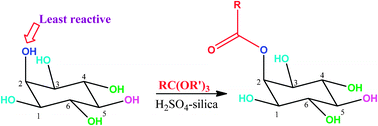
Chem Comm paper
Posted in Methodology, Regioselective | Leave a Comment »
Posted by naturalproductman on October 23, 2011
Pilar López-Ram-de-Víu and co-workers have reported in Organic and Biomolecular Chemistry on an epoxide opening reaction using a Grignard reagent – and the regioselectivity is switched when they use it in the presence of copper bromide.
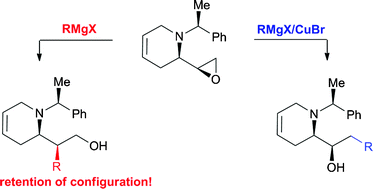
OBC paper
Posted in Copper, Grignard, Methodology, Named Reactions, Named Reagents, Regioselective, Ring Opening, Transition Metal | Leave a Comment »
Posted by naturalproductman on June 10, 2011
Hiroaki Ohno and co-workers from Kyoto University have reported in JOC on their total synthesis of lysergic acid via a regioselective epoxide opening using a reducing agent.

JOC paper
Posted in Alkaloids, Indoles, Methodology, Regioselective, Ring Opening, Transition Metal, Zinc | Leave a Comment »
Posted by naturalproductman on January 28, 2011
Gunter Helmchen Organisch-Chemisches Institut der Ruprecht-Karls-Universitat Heidelberg in Germany have published in JACS on an asymmetric allylic hydroxylation reaction starting from an allylic bicarbonate.

JACS paper
Posted in Asymmetric, Cascade Reactions, Iridium, Methodology, Regioselective, Transition Metal | Leave a Comment »
Posted by naturalproductman on October 19, 2010
Stephen Martin and co-workers have published in JACS on the synthesis of isokidamycin. They used a silicon tether to perform a regioselective Diels-Alder reaction.

JACS paper
Posted in Cycloaddition, Diels-Alder, Named Reactions, Pericyclic reactions, Polyketides, Regioselective, Ring forming | Leave a Comment »


