Michael Taylor and co-workers from the University of Wyoming reported in JACS on a method to label tryptophan residues using an N-carbamoyl pyridinium salt and light.
Archive for the ‘Light Mediated’ Category
Tryptophan labeling
Posted by naturalproductman on September 6, 2020
Posted in Light Mediated, Methodology, Proteins | Leave a Comment »
Zephycarinatines syntheses
Posted by naturalproductman on August 17, 2020
Hiroaki Ohno and co-workers at Kyoto University have reported in ACIE on the synthesis of zephycarinatines.
Posted in Alkaloids, Light Mediated, Total Synthesis | Leave a Comment »
JOC papers of interest 11/24/2015
Posted by naturalproductman on November 24, 2015
James White and co-workers reported on the synthesis of Huperzine A.
Scott Denmark and colleague reported on an asymmetrice 2,3-Wittig rearrangement.

Posted in Alkaloids, Light Mediated, Mechanistic, Methodology, Sigmatropic Rearrangements, Total Synthesis | Leave a Comment »
Triazoline + light
Posted by naturalproductman on September 4, 2015
Miguel Garcia-Garibay and co-workers at UCLA have reported in Organic Letters on a denitrogenation reaction of a triazoline using light to make an aziridine.

OL paper
Posted in Computational, Light Mediated, Mechanistic, Methodology | Leave a Comment »
Photoredox cross-coupling
Posted by naturalproductman on August 31, 2015
Larry Overman and co-workers reported in JACS on a photoredox coupling between tertiary alcohols and electron deficient olefins.
Posted in cross coupling, Light Mediated, Methodology | Leave a Comment »
Blue-LED induced cascade
Posted by naturalproductman on July 17, 2015
Zhiyong Jiang and co-workers from Henan University have reported in ACIE on a radical cascade cyclization using blue light emitted diodes.

Posted in Cascade Reactions, Light Mediated, Methodology, Radical Chemistry | Leave a Comment »
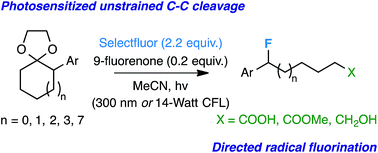
C-C bond cleavage of alpha-aryl acetals
Posted by naturalproductman on July 13, 2015
Thomas Lectka and co-workers from Johns Hopkins University have reported in Chemical Science on an interesting C-C bond cleavage process.
Posted in C-C Bond Breaking, Cascade Reactions, Light Mediated, Methodology | Leave a Comment »
Combining 2 concepts from sophmore organic chemistry (Sn2 and radical initiation)d
Posted by naturalproductman on July 11, 2015
The Finkesltein reaction is a straightforward Sn2 reaction – however, you don’t normally think of displacing an aromatic halide as an Sn2 reaction. Chao-Jun Li and co-workers at McGill University reported on the aromatic Finkelstein reaction using light.

Posted in Light Mediated, Methodology, Named Reactions, Radical Chemistry | Leave a Comment »