Yongjia Shang and co-workers from Anhui Normal University have reported in JOC on an interesting iron catalyzed cascade reaction.
Archive for the ‘Claisen rearrangement’ Category
Claisen-Diels-Alder cascade to spirooliganones A and B
Posted by naturalproductman on July 16, 2014
Rongbiao Tong and co-workers from the Hong Kong University of Science and Technology have reported in Org Lett on a slick reaction to form spirooliganones.

Posted in Cascade Reactions, Claisen rearrangement, Diels-Alder, Methodology, Named Reactions, Total Synthesis | Leave a Comment »
Ireland Claisen/Cope rearrangement
Posted by naturalproductman on June 30, 2014
James Leighton and co-workers reported in JACS on a mechanistic study of a tandem Ireland Claisen/Cope rearrangement reaction to form some polycyclic rings.

Posted in Cascade Reactions, Claisen rearrangement, Cope rearrangement, Mechanistic, Methodology, Named Reactions, oxy-Cope rearrangement | Leave a Comment »
Transtaganolide syntheses
Posted by naturalproductman on May 17, 2013
Brian Stoltz and co-workers have reported in ACIE on the syntheses of transtaganolides through an Ireland-Claisen rearrangement/Diels-Alder cascade.

Posted in Asymmetric, Cascade Reactions, Claisen rearrangement, Diels-Alder, Methodology, Named Reactions, Sesquiterpenes, Total Synthesis | Leave a Comment »
Vinigrol synthesis
Posted by naturalproductman on January 19, 2012
Louis Barriault and co-workers at the University of Ottawa have reported in ACIEE on the formal synthesis of vinigrol – I think this makes the number of groups to synthesize this molecule to 2, Baran being the other one.

Posted in Cascade Reactions, Claisen rearrangement, Diels-Alder, Methodology, Named Reactions, Total Synthesis | Leave a Comment »
Lysidicin A synthesis from a triple Claisen rearrangement
Posted by naturalproductman on January 17, 2012
Hidenori Watanabe and co-workers at the University of Tokyo have reported in Tetrahedron on the synthesis of lysidicin. The key reaction being a triple Claisen rearrangement that formed three of the aryl carbon bonds from aryl ether linkages.

Posted in Cascade Reactions, Claisen rearrangement, Methodology, Mitsunobu, Named Reactions, Polyketides, Total Synthesis | Leave a Comment »
Saudin synthesis
Posted by naturalproductman on November 21, 2011
Robert Boeckman and co-workers at the University of Rochester have reported in Tetrahedron on the synthesis of saudin.

Posted in Asymmetric, Cascade Reactions, Claisen rearrangement, Methodology, Named Reactions, Terpenes | Leave a Comment »
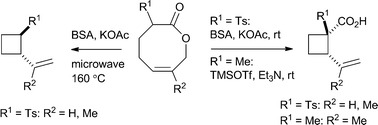
Transannular Claisen rearrangement
Posted by naturalproductman on October 23, 2011
Donald Craig and co-workers at Imperial College have reported in Organic and Biomolecular Chemistry on a transannular Claisen rearrangement converting a lactone into a cyclobutane.
Posted in Claisen rearrangement, Methodology, Named Reactions, Ring contraction | Leave a Comment »